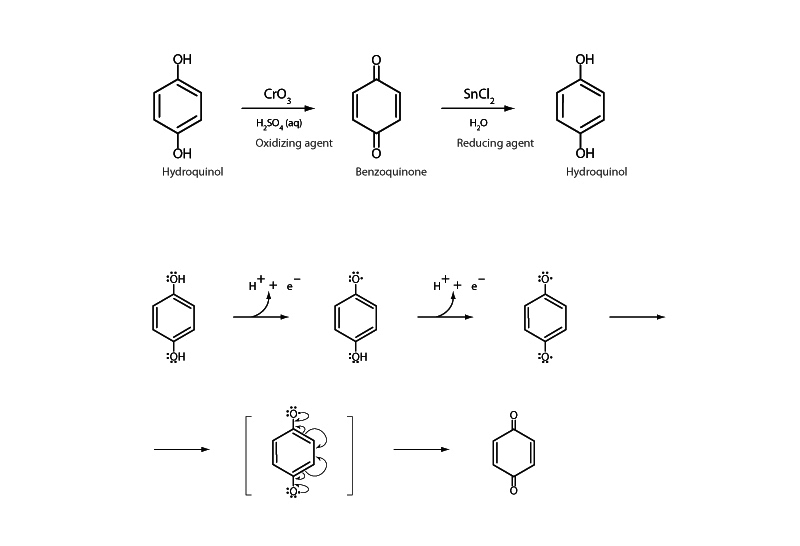
Hydroquinol is a type of phenol with hydroxyl groups bonded to a benzene ring in the para position. Hydroquinol is reversibly oxidized to the dicarbonyl compound benzoquinone. A quinol represents the reduced end of a redox couple with a quinone. While hydroquinol is aromatic, benzoquinone is merely conjugated, so the powerful reduction potential of oxygen favoring oxidation is balanced by the loss of the aromaticity in the quinol. This balancing act explains why the reaction is reversible.
The mechanism for the oxidation of hydroquinol passes through a free radical intermediate, a semiquinone, on the way to benzoquinone. Semiquinones are relatively stable free radicals due to resonance stabilization. The formation of the semiquinone free radical intermediate promotes one of the most important aspects of the oxidation of a quinol to a quinone, that it occurs with the transfer of a single electron at a time.
