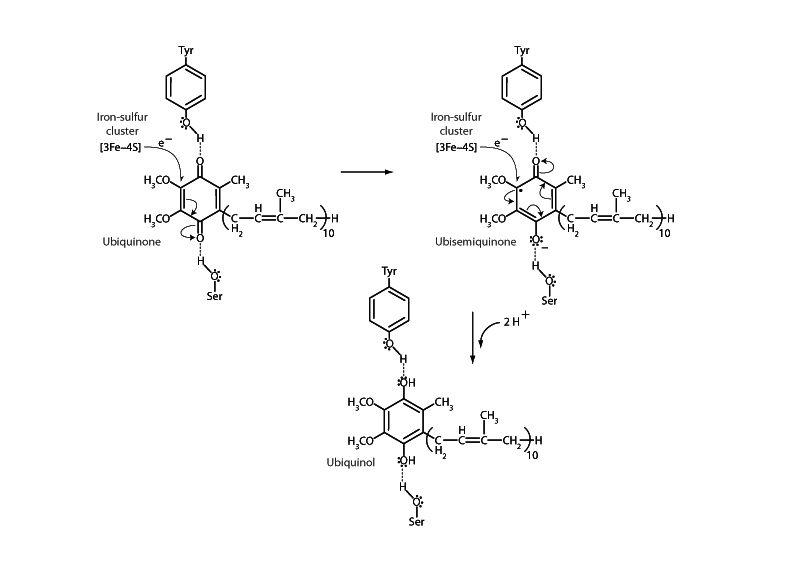
Coenzyme Q10, also called ubiquinone, is a lipid soluble, mobile electron carrier present in high concentrations on the inner membrane of the mitochondrion. Ubiquinone plays a crucial role in the electron trans- port chain of aerobic metabolism, carrying electrons from enzyme complexes I and II to complex III.
Ubiquinone reduction proceeds through two single electron transfers. Receiving its first electron ubiquinone becomes the ubisemiquinone radical. Semiquinones are relatively stable free radicals due to resonance stabilization. Receipt of the second electron forms the fully reduced form of Coenzyme Q10, ubiquinol, which possesses two hydroxyl groups bonded to an aromatic ring.
The figure above shows the reduction of Coenzyme Q10 by an iron-sulfur cluster of succinate dehydrogenase (complex II). Following the first single electron reduction step, a semiquinone radical species is formed. A second electron arrives from the [3Fe-4S] cluster to provide full reduction to ubiquinol. Note that ubiquinone and iron-sulfur clusters carry out single electron transfers. This is different than the electron carrier NADH which transfers 2 electrons. In the electron transport system, these double and single electron transfers are intermediated by a flavin, FADH2 or FMN, which can perform both types.