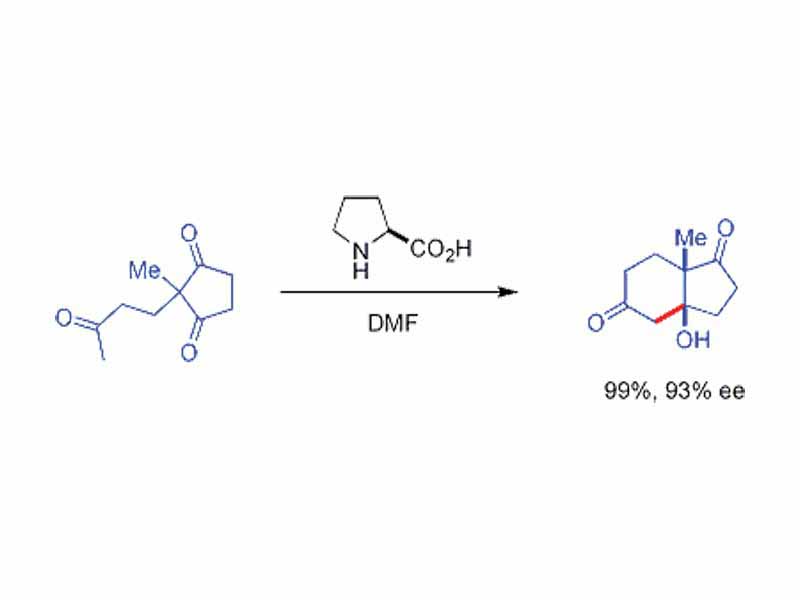
The use of chiral secondary amine catalysts in aldol reaction. These secondary amines form transient enamines when exposed to ketones, which may react enantioselectively with suitable aldehyde electrophiles. This is known as enamine catalysis, a type of organocatalysis, since the catalyst is entirely based on a small organic molecule. In a seminal example, proline efficiently catalyzed the cyclization of a triketone:
Click this LINK to visit the original image and attribution information. Right click on the image to save the 800px teaching JPEG.