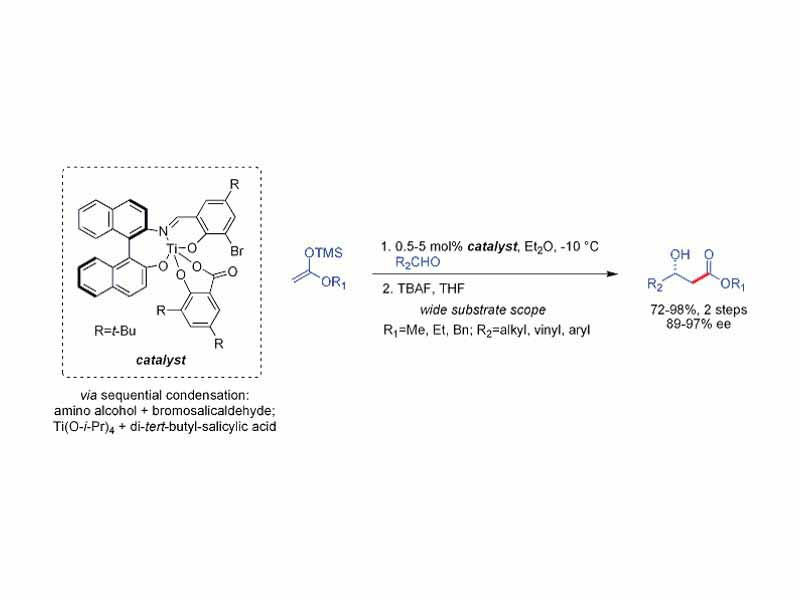
The Mukaiyama aldol reaction is the nucleophilic addition of silyl enol ethers to aldehydes catalyzed by a Lewis acid such as boron trifluoride or titanium chloride. The Mukaiyama aldol reaction does not follow the Zimmerman-Traxler model. Carreira has described particularly useful asymmetric methodology with silyl ketene acetals, noteworthy for its high levels of enantioselectivity and wide substrate scope.
Click this LINK to visit the original image and attribution information. Right click on the image to save the 800px teaching JPEG.