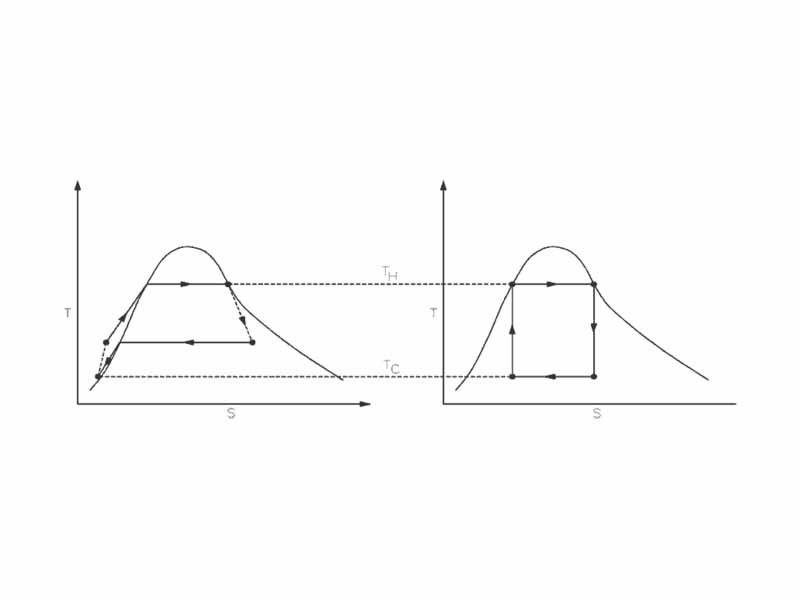
A real engine (left) compared to the Carnot cycle (right). The entropy of a real material changes with temperature. This change is indicated by the curve on a T-S diagram. For this figure, the curve indicates a vapor-liquid equilibrium (See Rankine cycle). Irreversible systems and losses of heat (for example, due to friction) prevent the ideal from taking place at every step.
Click this LINK to visit the original image and attribution information. Right click on the image to save the 800px teaching JPEG.