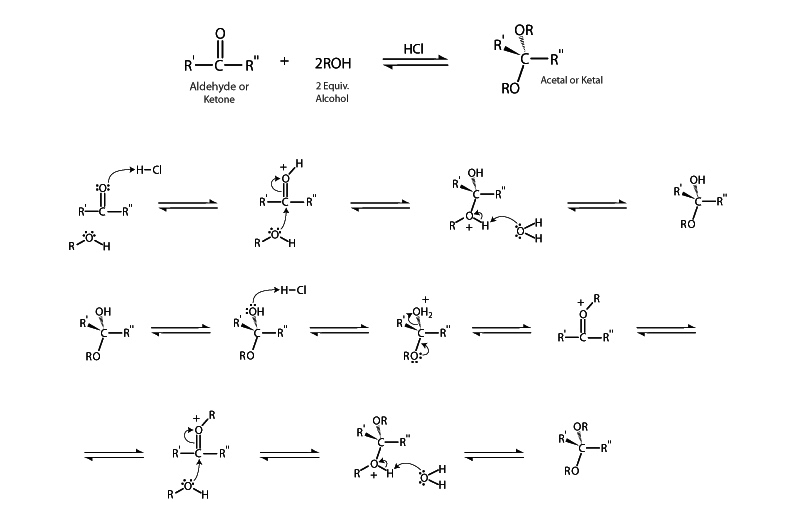
Acetal formation is the quintessential representative of an important class of reactions, nucleophilic additions, in which a nucleophile attaches to a carbonyl carbon. The greater electronegativity of oxygen over carbon in the carbonyl group makes carbonyl carbon somewhat electron deficient. This is why carbonyl compounds are subject to nucleophilic addition. A nucleophile is a reagent that can supply an electron pair to form a new bond, allowing the carbonyl carbon needs to resolve itself in a thermodynamically favorable direction. In acetal formation, nucleophilic addition happens twice. The first addition forms the simple addition product, a hemiacetal. Following dehydration of the hemiacetal, a second addition of alcohol forms the acetal.
The reaction mechanism in acetal formation begins with protonation of the ketone or aldehyde carbonyl oxygen by an acid catalyst. Protonation of oxygen increases the attractiveness of the carbonyl group carbon, making it even more electropositive. Next, dehydration of the tetrahedral intermediate forms an oxonium cation which is then approached by another alcohol nucleophile. This forms the acetal. On the bench top, acetal formation is often employed as a strategy for protecting carbonyl groups from hostile reaction conditions.
