Interdisciplinary Note (1 of 12)
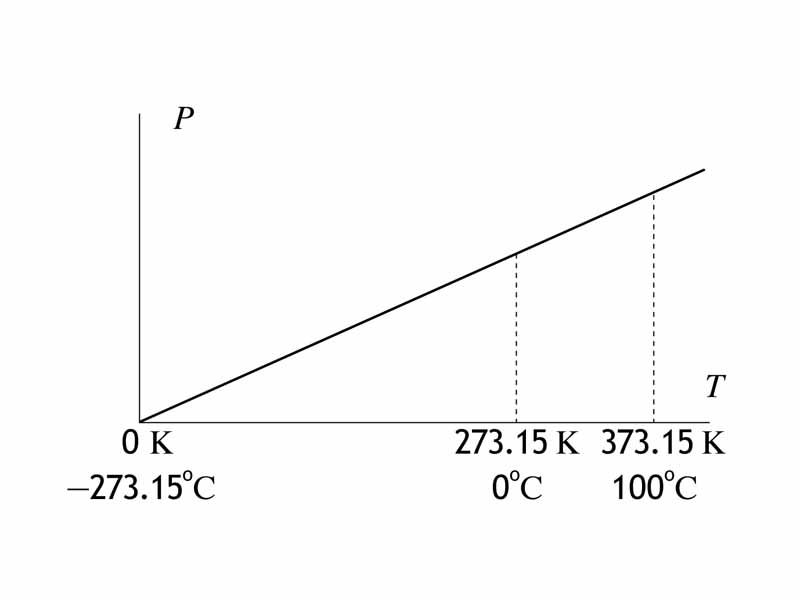
For thermodynamics problem solving, remember that you often need to use the Kelvin scale of temperature. One of the wrong answers in multiple choice gas laws problem is always the answer you derive if you use the Celsius scale by mistake.
The temperature in Kelvin is the true thermodynamic temperature, giving you insight into the properties of an ideal gas at both at the macrostate level and at the particle level. The macrostate refers to properties accessible from the point of view of benchtop observation, such as the pressure, volume and temperature. The particle level is the domain of kinetic theory where we see the particles having a distribution of velocities.
For an ideal gas, the temperature on the Kelvin scale is proportional to the product of the pressure and the volume. The Kelvin scale can be obtained via the equation of state for the ideal gas (the ideal gas law) from pressure and volume. Furthermore, because the internal energy of a given ideal gas is proportional to its temperature, we can use the Kelvin temperature change to indicate internal energy change. If the temperature has increased for an ideal gas, the internal energy has increased. For a sample of ideal gas, viewed at the particle level, the Kelvin temperature describes the average kinetic energy of the particles.