Interdisciplinary Note (25 of 26)
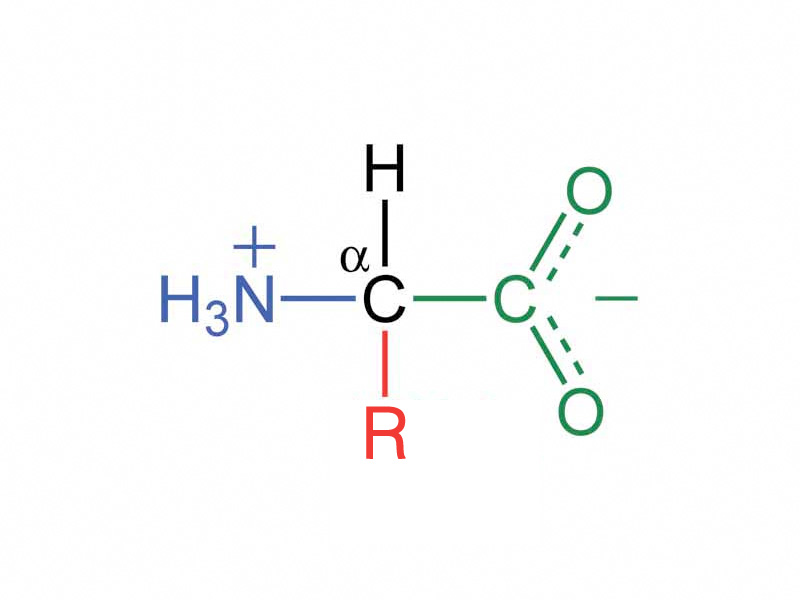
Pay attention to the titration of amino acids. Most of the amino acids possess two ionizable groups, a carboxyl group and an amine group on the central carbon. You refer to these as the α-carboxyl and α-amine groups respectively. When we talk about titrating an amino acid, we're not talking about residues that are part of a polypeptide. We're talking about the free amino acid in aqueous solution. What changes will it undergo as we raise the pH slowly pH = 1 to pH = 13? There's variation, but the typical α-carboxyl group will have a pKa ranging usually a bit above 2.0, but sometimes a little below that. The α-amine group pKa will be around 9.5.
Seven of the amino acids also possess an ionizable side chain in addition to the two groups on the central carbon. Aspartic acid and glutamic acid contain a carboxyl group. The pKa for those two is around 4.0. Because the pKa is below physiological pH, their carboxylate forms will predominate at physiological pH, which is why people almost never say "aspartic acid" or "glutamic acid" but, instead, "aspartate" and "glutamate". Two amino acids contain basic side chains. Lysine has an amine group, pKa = 10.5. The arginine side chain has a side chain that looks like the imine form of urea, which is called guanidinium, pKa = 12.0. When histidine ionizes, it will be protonated too. It's side chain is a ring with nitrogen heteroatoms known as imidizole (remember this one!). Unlike lysine and arginine, with a pKa of 6.1, the imidizole ring of histidine is only protonated 1/20 of the time. They are reliable positives, but it is not. Protons come and go from histidine, and it's pKa has a variability within the microenvironment of a folded protein. Histidine is the quintessential acid-base catalyst in enzyme mechanisms. Cysteine has a sulfhydral group (pKa = 8.1) and tyrosine has a phenolic hydroxyl group (pKa - 10.5), so that rounds out the amino acids with ionizable side chains.
The curves describing the titration of a simple amino acid, beginning with an acidic solution, adding strong base dropwise, correspond to a fully protonated start, losing each proton in step-wise fashion. There is pH at which not only does the predominate form of the molecule carry no net electric charge, but the probability of variation in either direction is exactly equal, so it does not migrate if electric potential is imposed on the solution. This is the isoelectric point. For the plain jane amino acids, this will be the simple average of the two respective pKa's of its amine and carboxyl groups (that way, the chance the amine will be deprotonated and neutral will exactly equal the chance the carboxyl will be protonated and neutral). The titration curves of amino acids with acidic or basic groups in their side chains have three steps. To find the pKa of those, the rule-of-thumb is to "average the two like groups". For example, with lysine, we average the pKa's of the two amine groups (approximately 10.5 and 9.5), so the isoelectric point, pI, for lysine is very near to 10.0. At that pH the carboxyl group is definitely negative, and the chance that the higher pKa amine is actually neutral and not positive is balanced by the chance the lower pKa amine is actually positive and not neutral, so there will be a 100% chance of that side of things being a +1 charge to balance the carboxyl group. That may take a bit of rumination.