Interdisciplinary Note (26 of 26)
Let's take the concept of isoelectric point one step further. It's an important point that the concept of isoelectric point doesn't only apply to a single free amino acid in solution. An entire protein will also possess an isoelectric point. Even though there may easily be dozens or more ionizable side chains on an average sized protein, there will still be a particular pH where the most likely form will be electrically neutral, and the probability of variation in either direction will be balanced. Within a pH environment equal to its isoelectric point, pI, that particular protein will not migrate under an electric field.
The isoelectric point of a protein is a meaningful signifier. It tells you some important things about the protein right off. Histones, the proteins that constitute the nucleosome cores of chromatin, for example, have an especially high isoelectric point. You know right from this fact that histones must have plenty of lysine and arginine residues. In fact, the enzyme histone acetyl transferase acetylates specific lysine residues on histones to them into amides, which are neutral. This is a big topic for gene expression because it has important consequences for the conversion of transcriptionally inactive condensed chromatin (heterochromatin) into transcriptionally active euchromatin. The world of chromatin modeling is one of the great rabbit holes of molecular biology where even the complexities of complexities have complexities. For now, it suffices to say that with a high pI, a protein is going to be positively charged at physiological pH. In other words, you can use the isoelectric point of a protein to predict the direction of the overall charge. The isoelectric point represents the inflection where the protein would transition from net positive to net negative under titration with base. You'd have to put a histone into a high pH environment to get it to be electrically neutral.
Isoelectric focusing is an important technique in the protein lab that takes advantage of the variation in isoelectric point among proteins as the basis for a method of analytical or preparative separation. An isoelectric strip is a solid support on which there is an emulsion through which proteins can migrate. The emulsion is designed so that there is a fixed pH gradient from one end of the strip to the other. If you place a sample mixture of proteins at the acidic end, they are all going to be positively charged. The pH is below all of their isoelectric points. Place that end of the strip near the anode in your device (the anode is the positive terminal; lab equipment uses the electrolytic cell convention, not the galvanic cell convention for assigning the names anode and cathode, so the anode is the positive terminal). Next to the positive terminal, the proteins, which are all positively charged, being at the low pH end of the isoelectric strip, will begin to migrate into the strip. The pH starts to rise as they move across the strip. Soon, a protein with a low isoelectric point (it must have lots of aspartate and glutamate) reaches a pH equal to its isoelectric point, so it stops moving, but the rest of the proteins keep going. One by one they all drop out of the race until there are only a few proteins left still moving, proteins with high pI like histones, but when a pH is finally reached equal to their isoelectric point, they stop too. That's isoelectric focusing.
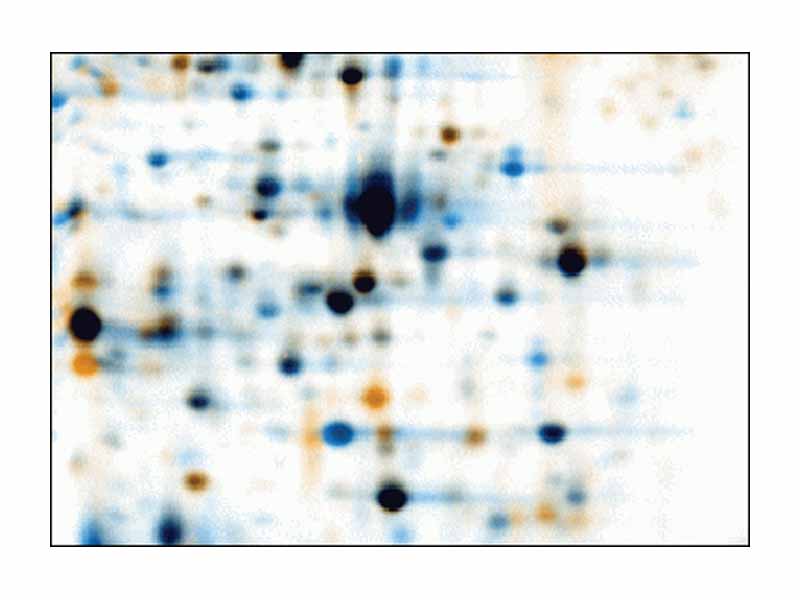
A big application for isoelectric focusing would be as a next step to take the strip and use it like sample wells for SDS-PAGE (sodium dodecyl sulfate - polyacrylamide gel electrophoresis). This is called two dimensional electrophoresis. From left to right on the gel, the proteins are separated by isoelectric point. From top to bottom, the proteins are separated by size. This is valuable for assaying the proteome of a cell because you can visualize many more proteins at at time than on a standard SDS-PAGE gel.