Interdisciplinary Note (24 of 26)
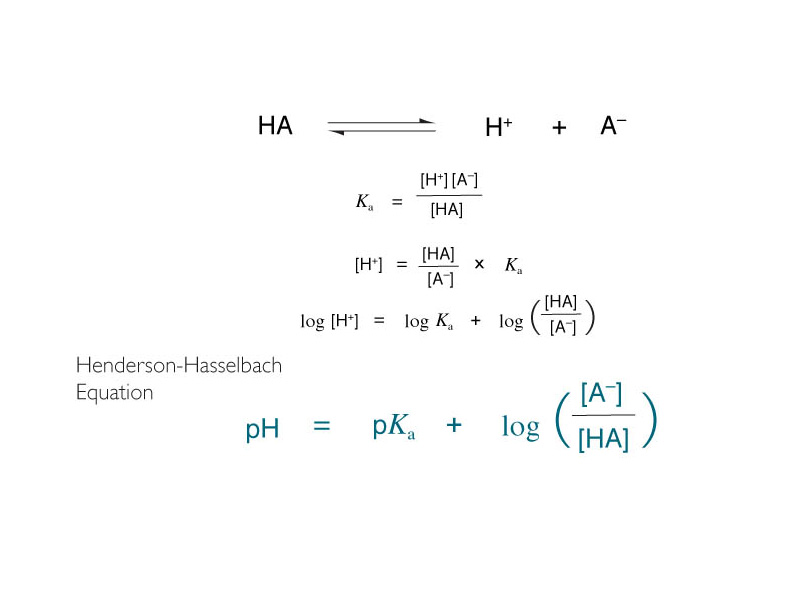
The Henderson-Hasselbalch equation is absolutely crucial for the MCAT. In Chem 101, we learn to use the Henderson-Hasselbalch equation in a 'straight-ahead' manner. We learn to look at it as a function machine that tells us the pH of a buffer solution as a function of the pKa of the acid form and the ratio of the concentrations of conjugate base to acid in the solution.
However, in biochemistry especially, is it absolutely central to be thinking of this equation the other way around. The equation will tell you the ionization state of solution components within a dictated pH environment. In physiology, pH is determined by over-riding buffer systems that maintain homeostasis. The pH is not a variable except within a very narrow range. Think of how the Henderson-Hasselbalch equation can be made to work the other way around. The state of ionization of an ionizable group on a biomolecule depends on how its pKa compares to the pH of its environment.
Picture the side-chain of an amino acid lysine. Can you picture it? A long hydrocarbon chain with a terminal primary amine. Lysine side chain amine group has a pKa near 10.5. Which form is predominating at physiological pH? The unprotonated amine group (A- in the equation, and no the amine is not negatively charged. It's the conjugate base.) or is it the protonated, positively charged ammonium? (the conjugate acid HA in the equation) If there is a physiological pH of 7.4, the Henderson-Hasselbalch equation tells you that the ammonium ion form will predominate at a ratio near to 1000 to 1. 7.4 = 10.5 + log ([A-]/[HA]). log (1/1000) = -3, so ammonium predominates.
Think of the pKa as telling you how a group feels about its surroundings. If it finds itself in an aqueous solution with a pH lower than its pKa, the world around feels like a proton pressure. Protons will pile onto it in exponential base 10 increase for for every point its pKa is above the pH. That's how the lysine side chain with a pKa of 10.5 feels in a pH 7.4 environment. There is a proton pressure from the surrounding solution environment onto the amine group, and it will spend almost all its time in the protonated ammonium form. The equation solves with a ratio (1:1000) deprotonated to protonated. We know that lysine side chain is a reliable positive charge at physiological pH.
However, instead of lysine, let's put an aspartate side chain into the same environment. Can you picture the side chain of aspartate? Aspartate side chain has a two carbon hydrocarbon stretch off of the central carbon and then a carboxyl group whose pKa is 3.9. Physiological pH is higher than the pKa of aspartate's carboxyl group side chain, so the same world that feels like a proton pressure to lysine feels like a proton suction to aspartate. The surroundings are pulling protons off of it. Protons fall downhill in free energy from the aqueous environment onto lysine at pH 7.4 but they will be falling off from aspartate onto the water at 7.4. The protons are always settling towards equilibrium. Because physiological pH is 3.5 units higher than aspartate's pKa (7.4 vs. 3.9), the carboxyl group will be deprotonated at a ratio of approximately 3000:1. (Just interpolate these in between logs if they make your brain hurt. The MCAT would probably not ding you if you said 5000:1.) In other words, the deprotonated, carboxylate form of the aspartate side chain predominates at physiological pH, so aspartate is a reliable negative in biochemistry.
The mind of AAMC is bent on the Henderson-Hasselbach equation. They know that for you to do well in medical school biochemistry, it needs to become as natural as breathing.