Interdisciplinary Note (19 of 22)
Let us discuss conformational changes in the thermodynamic context a bit further to continue in our preview of some important ideas which will be coming soon as our MCAT course moves into thermodynamics. As a mental exercise, imagine that all of the molecules in a sample of a pure substance assumed one of the higher energy conformations.
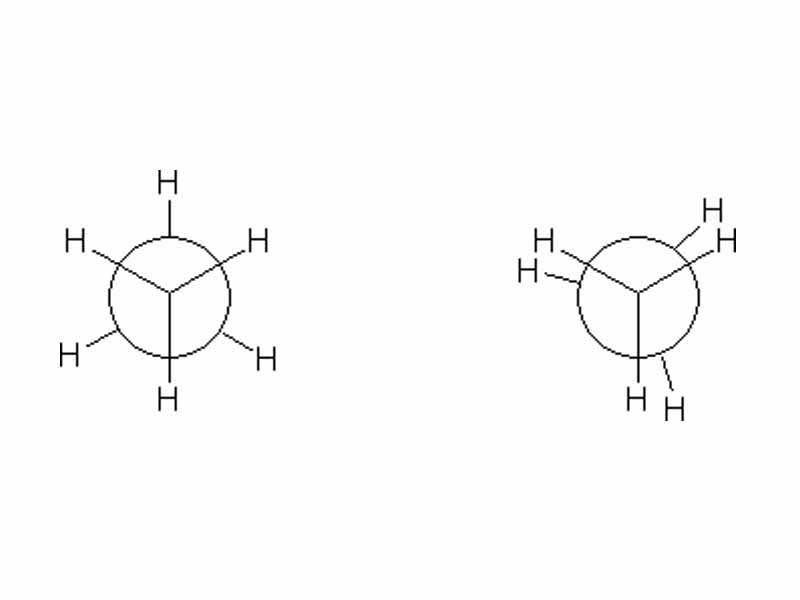
For example, imagine the all of the molecules within a sample of ethane in the staggered conformation. Now imagine them rotating to assume the eclipsed conformation. What has happened to the total internal energy of the sample? The answer is that it has increased.
The electrostatic potential energy has increased at the particle level. Would heat need to flow in or would heat flow out of the system if all the ethane molecules underwent rotation of from the staggered to the eclipsed conformation? Because volume change is not a factor (there is no pressure volume work) so the answer is that heat would flow in to match the internal energy change. Internal energy change, as we have said, maps back to electrostatic potential energy.
Now imagine if all of the molecules were rotated back to the staggered form. The electrostatic potential energy decreased, the internal energy decreased. Where did the energy go? The answer is negative enthalpy change, i.e. heat flowed out into the surroundings.
Now ask yourself, which direction is more likely? Just like dropping a book on the floor and hearing the sound is more likely than the sound coming back and lifting the book back onto the table, the answer is that the direction in which heat flows out from system into the surroundings and dissipates is more likely. This increases the entropy of the surroundings.
One final note. Although the state in which all of the molecules are in the staggered state is more likely than the state in which all the molecules are in the eclipsed state, neither state is the most likely state for the system of ethane molecules. The most likely state is the one in which the vast majority of molecules are in the staggered state with a few in the eclipsed state. At that state, the likelihood of a bit of heat coaelescing in the system equals the likelihood of a bit of heat dissipating from the system. The rate forward is the same as the rate backward. The system is in equilibrium.