Interdisciplinary Note (21 of 22)
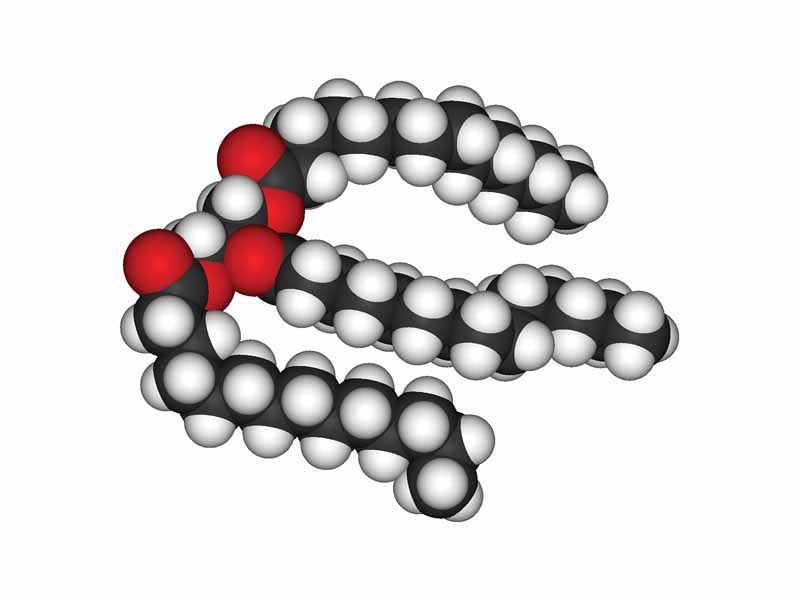
The longer and more saturated the fatty acid chains of a triglyceride, the higher will be the melting point of the fat. These characteristics enable the maximum Van der Waals interaction between the hydrocarbon chains, meaning that each chain experiences a higher net electrostatic force of attraction to its neighbor.
Therefore, the transition from solid to liquid, in which the chains draw apart and allow much more free rotation around bond axes, requires greater thermal energy. With saturated fats, the phase transition represents a larger increase in electrostatic potential energy as the straighter chains pull away from each other.